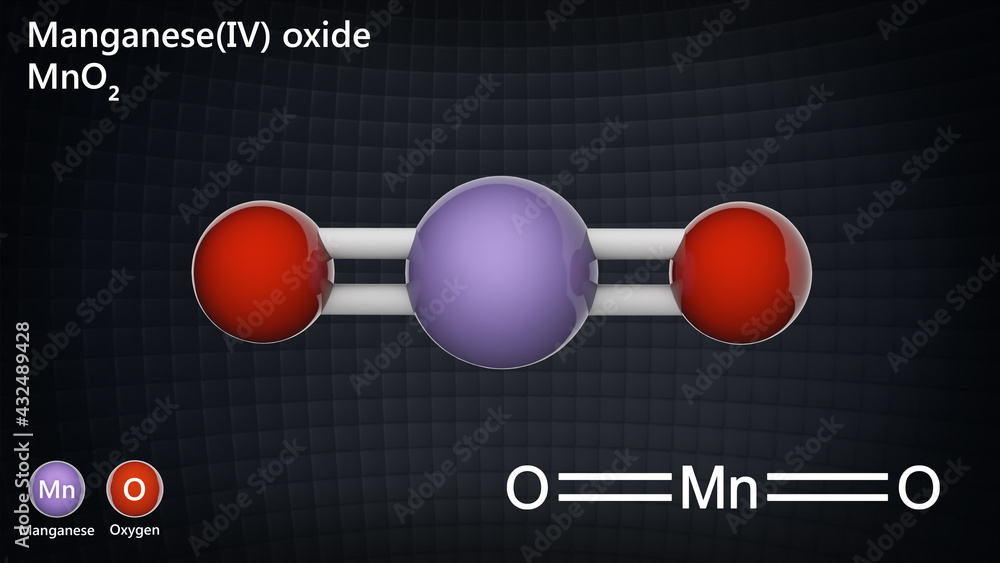
Manganese (Iv) Oxide Ionic Or Covalent . Write the formulas of the following ionic compounds: A) nacl b) albr3 c) bah2. A) identify the cation and anion. Find out how potassium manganate. Any sample of manganese (iv) oxide will have many, many,. Find out the names of common cations and anions, including manganese (ii) and manganese (iii) ions. Bromine commonly forms an anion. Calcium commonly forms a cation with a charge of +2. That is, does it have ionic bonds, or covalent bonds?. The roman numeral iv in. When writing an ionic formula, the simplest ratio is the 'ionic formula.'. Na is a group 1 metal, and thus it forms the cation na +, called “sodium”. (a) chromium(iii) phosphide (b) mercury(ii) sulfide (c) manganese(ii) phosphate. Learn about the reactions and properties of manganese (ii) and manganese (vii) ions in solution. Name the following ionic compounds:
from stock.adobe.com
Name the following ionic compounds: Calcium commonly forms a cation with a charge of +2. A) nacl b) albr3 c) bah2. Learn about the reactions and properties of manganese (ii) and manganese (vii) ions in solution. The roman numeral iv in. Bromine commonly forms an anion. Any sample of manganese (iv) oxide will have many, many,. That is, does it have ionic bonds, or covalent bonds?. (a) chromium(iii) phosphide (b) mercury(ii) sulfide (c) manganese(ii) phosphate. Find out how potassium manganate.
Manganese(IV) oxide (or Manganese dioxide) is the compound
Manganese (Iv) Oxide Ionic Or Covalent Na is a group 1 metal, and thus it forms the cation na +, called “sodium”. A) nacl b) albr3 c) bah2. Any sample of manganese (iv) oxide will have many, many,. Find out how potassium manganate. The first question we ask is if the compound is ionic or covalent? (a) chromium(iii) phosphide (b) mercury(ii) sulfide (c) manganese(ii) phosphate. Write the formulas of the following ionic compounds: To write the chemical formula for manganese (iv) oxide, you must make sure to balance the charges. Learn about the reactions and properties of manganese (ii) and manganese (vii) ions in solution. Name the following ionic compounds: Calcium commonly forms a cation with a charge of +2. Bromine commonly forms an anion. Find out the names of common cations and anions, including manganese (ii) and manganese (iii) ions. The roman numeral iv in. Learn how to name ionic compounds using systematic and common names. When writing an ionic formula, the simplest ratio is the 'ionic formula.'.
From chemcraft.su
Manganese(IV) oxide, >85 (pure p.a.) chemcraft.su Manganese (Iv) Oxide Ionic Or Covalent When writing an ionic formula, the simplest ratio is the 'ionic formula.'. Learn how to name ionic compounds using systematic and common names. Calcium commonly forms a cation with a charge of +2. To write the chemical formula for manganese (iv) oxide, you must make sure to balance the charges. Learn about the reactions and properties of manganese (ii) and. Manganese (Iv) Oxide Ionic Or Covalent.
From chemwiki.ucdavis.edu
Acids, Bases and Solvent Systems Chemwiki Manganese (Iv) Oxide Ionic Or Covalent (a) chromium(iii) phosphide (b) mercury(ii) sulfide (c) manganese(ii) phosphate. Bromine commonly forms an anion. Find out the names of common cations and anions, including manganese (ii) and manganese (iii) ions. Any sample of manganese (iv) oxide will have many, many,. When writing an ionic formula, the simplest ratio is the 'ionic formula.'. The first question we ask is if the. Manganese (Iv) Oxide Ionic Or Covalent.
From stock.adobe.com
Manganese(IV) oxide (or Manganese dioxide) is the compound Manganese (Iv) Oxide Ionic Or Covalent Na is a group 1 metal, and thus it forms the cation na +, called “sodium”. When writing an ionic formula, the simplest ratio is the 'ionic formula.'. Name the following ionic compounds: That is, does it have ionic bonds, or covalent bonds?. Find out the names of common cations and anions, including manganese (ii) and manganese (iii) ions. Find. Manganese (Iv) Oxide Ionic Or Covalent.
From www.slideserve.com
PPT Naming Multivalent Compounds PowerPoint Presentation, free Manganese (Iv) Oxide Ionic Or Covalent Find out the names of common cations and anions, including manganese (ii) and manganese (iii) ions. Learn about the reactions and properties of manganese (ii) and manganese (vii) ions in solution. Na is a group 1 metal, and thus it forms the cation na +, called “sodium”. Bromine commonly forms an anion. Write the formulas of the following ionic compounds:. Manganese (Iv) Oxide Ionic Or Covalent.
From www.scienceabc.com
Manganese Oxide Chemical Formula, Properties And Uses » ScienceABC Manganese (Iv) Oxide Ionic Or Covalent Bromine commonly forms an anion. Any sample of manganese (iv) oxide will have many, many,. A) identify the cation and anion. Find out the names of common cations and anions, including manganese (ii) and manganese (iii) ions. That is, does it have ionic bonds, or covalent bonds?. When writing an ionic formula, the simplest ratio is the 'ionic formula.'. To. Manganese (Iv) Oxide Ionic Or Covalent.
From www.dreamstime.com
Manganese Dioxide Chemical Formula Structure Design Stock Illustration Manganese (Iv) Oxide Ionic Or Covalent Name the following ionic compounds: Bromine commonly forms an anion. Na is a group 1 metal, and thus it forms the cation na +, called “sodium”. Write the formulas of the following ionic compounds: A) nacl b) albr3 c) bah2. Find out the names of common cations and anions, including manganese (ii) and manganese (iii) ions. Learn how to name. Manganese (Iv) Oxide Ionic Or Covalent.
From www.lazada.com.my
Manganese (IV) Oxide CP, 500g, HmbG Lazada Manganese (Iv) Oxide Ionic Or Covalent Name the following ionic compounds: Learn about the reactions and properties of manganese (ii) and manganese (vii) ions in solution. When writing an ionic formula, the simplest ratio is the 'ionic formula.'. That is, does it have ionic bonds, or covalent bonds?. A) nacl b) albr3 c) bah2. To write the chemical formula for manganese (iv) oxide, you must make. Manganese (Iv) Oxide Ionic Or Covalent.
From www.youtube.com
How to Write the Formula for Manganese (IV) oxide YouTube Manganese (Iv) Oxide Ionic Or Covalent To write the chemical formula for manganese (iv) oxide, you must make sure to balance the charges. The first question we ask is if the compound is ionic or covalent? That is, does it have ionic bonds, or covalent bonds?. Find out how potassium manganate. Bromine commonly forms an anion. Name the following ionic compounds: A) identify the cation and. Manganese (Iv) Oxide Ionic Or Covalent.
From www.apcpure.com
Manganese (IV) Oxide 75 APC Pure Manganese (Iv) Oxide Ionic Or Covalent A) nacl b) albr3 c) bah2. The first question we ask is if the compound is ionic or covalent? Write the formulas of the following ionic compounds: To write the chemical formula for manganese (iv) oxide, you must make sure to balance the charges. Learn how to name ionic compounds using systematic and common names. Any sample of manganese (iv). Manganese (Iv) Oxide Ionic Or Covalent.
From solvedlib.com
Manganese(IV) oxide reacts with aluminum to form elem… SolvedLib Manganese (Iv) Oxide Ionic Or Covalent Find out the names of common cations and anions, including manganese (ii) and manganese (iii) ions. Na is a group 1 metal, and thus it forms the cation na +, called “sodium”. Write the formulas of the following ionic compounds: Name the following ionic compounds: Find out how potassium manganate. Any sample of manganese (iv) oxide will have many, many,.. Manganese (Iv) Oxide Ionic Or Covalent.
From www.researchgate.net
Structures of selected manganese oxides (MnOx) featuring different Manganese (Iv) Oxide Ionic Or Covalent A) identify the cation and anion. A) nacl b) albr3 c) bah2. The first question we ask is if the compound is ionic or covalent? (a) chromium(iii) phosphide (b) mercury(ii) sulfide (c) manganese(ii) phosphate. Calcium commonly forms a cation with a charge of +2. When writing an ionic formula, the simplest ratio is the 'ionic formula.'. Find out how potassium. Manganese (Iv) Oxide Ionic Or Covalent.
From studylib.net
Nomenclature 1 Binary Ionic Compounds 1. Write the chemical Manganese (Iv) Oxide Ionic Or Covalent Find out how potassium manganate. A) nacl b) albr3 c) bah2. Na is a group 1 metal, and thus it forms the cation na +, called “sodium”. To write the chemical formula for manganese (iv) oxide, you must make sure to balance the charges. Write the formulas of the following ionic compounds: Find out the names of common cations and. Manganese (Iv) Oxide Ionic Or Covalent.
From www.scienceabc.com
Manganese Oxide Chemical Formula, Properties And Uses » ScienceABC Manganese (Iv) Oxide Ionic Or Covalent The roman numeral iv in. Any sample of manganese (iv) oxide will have many, many,. Bromine commonly forms an anion. To write the chemical formula for manganese (iv) oxide, you must make sure to balance the charges. A) nacl b) albr3 c) bah2. Learn about the reactions and properties of manganese (ii) and manganese (vii) ions in solution. Write the. Manganese (Iv) Oxide Ionic Or Covalent.
From segudanginformasibagus58.blogspot.com
Manganese Iv Ion Electron Configuration The ground state electron Manganese (Iv) Oxide Ionic Or Covalent Any sample of manganese (iv) oxide will have many, many,. Learn how to name ionic compounds using systematic and common names. Calcium commonly forms a cation with a charge of +2. Name the following ionic compounds: To write the chemical formula for manganese (iv) oxide, you must make sure to balance the charges. The roman numeral iv in. The first. Manganese (Iv) Oxide Ionic Or Covalent.
From pubs.acs.org
Hydrated Manganese(II) Phosphate (Mn3(PO4)2·3H2O) as a Water Oxidation Manganese (Iv) Oxide Ionic Or Covalent (a) chromium(iii) phosphide (b) mercury(ii) sulfide (c) manganese(ii) phosphate. When writing an ionic formula, the simplest ratio is the 'ionic formula.'. To write the chemical formula for manganese (iv) oxide, you must make sure to balance the charges. The roman numeral iv in. The first question we ask is if the compound is ionic or covalent? Write the formulas of. Manganese (Iv) Oxide Ionic Or Covalent.
From www.fishersci.pt
Manganese(IV) oxide, 88, electrolytically precipitated, active, ACROS Manganese (Iv) Oxide Ionic Or Covalent Learn how to name ionic compounds using systematic and common names. A) nacl b) albr3 c) bah2. That is, does it have ionic bonds, or covalent bonds?. Na is a group 1 metal, and thus it forms the cation na +, called “sodium”. Learn about the reactions and properties of manganese (ii) and manganese (vii) ions in solution. Name the. Manganese (Iv) Oxide Ionic Or Covalent.
From www.youtube.com
How to Write the Formula for Manganese (IV) carbonate YouTube Manganese (Iv) Oxide Ionic Or Covalent Calcium commonly forms a cation with a charge of +2. Na is a group 1 metal, and thus it forms the cation na +, called “sodium”. Learn about the reactions and properties of manganese (ii) and manganese (vii) ions in solution. Name the following ionic compounds: The roman numeral iv in. Bromine commonly forms an anion. A) identify the cation. Manganese (Iv) Oxide Ionic Or Covalent.
From pixels.com
Manganese (iv) Oxide Photograph by Andrew Lambert Photography Pixels Manganese (Iv) Oxide Ionic Or Covalent Any sample of manganese (iv) oxide will have many, many,. Name the following ionic compounds: Write the formulas of the following ionic compounds: Calcium commonly forms a cation with a charge of +2. Learn about the reactions and properties of manganese (ii) and manganese (vii) ions in solution. To write the chemical formula for manganese (iv) oxide, you must make. Manganese (Iv) Oxide Ionic Or Covalent.